Large Drip Tray | Effective Spill Containment Solutions - large drip tray
(1) Containers of liquid organic peroxide must be handled and transported with the top side indicated. Laying a container on its side or upside down is not permitted for safety assurance.
Organic peroxideexplosion
Danger signs are black, white and red. The word “DANGER” is written in white inside a red oval on a black background. The rest of the text and icons are printed in black on a white background.
No! You can order one sign, a pack of signs or even mass-produced signs — we’ll deliver the signs you need to make your workplace safer. Plus, we offer free shipping on orders over $100, so if you need a large number of signs, you’ll save on shipping! Alternatively, if your order is under $100, please enter your shipping details during the checkout process — we’ll provide a shipping quote on the spot.
•In case of fire As explosion can be anticipated in a fire, fire expansion must be prevented by pouring water over the surrounding area, and fire-fighting personnel should keep at a safe distance. Fire extinguishing agents: water jet, water spray, foams, and reinforcing liquid.
class 5.2:organic peroxideexamples
Danger signs on-site must be AS 1319-1994 compliant. This Australian Standard outlines the need to devise and implement safety signs in your work environment to ensure safe workplace behaviour, practices and hazards prevention.Danger signs (as well as most other safety signs) must be displayed in places where they attract maximum attention and the message must be simple and straightforward — the letters must be legible even from a reasonable distance away. Essentially, your danger signs should be unmissable! Your signs should ideally not be hanging from above. Signs must be still at all times to ensure employees, visitors and pedestrians can easily read the print. With this in mind, the preferred location to place a sign is on a wall — doors, racks, windows and other moving equipment are not suitable spots for a danger sign. If you have no other choice than to hang the sign, make sure that it’s not an obstruction for anyone passing by e.g. hanging the sign low so that people need to duck underneath or skirt around the sign. Again, this might cause employees, pedestrians and visitors to ignore the sign.
•In case of very small fire A foam fire extinguisher can be used. After extinguishing the fire, keep pouring water because the residual heat causes decomposition of organic peroxide generating white smoke. If water is poured on the fire at the outbreak of the fire, the fire cannot be extinguished.
(3) Activated-sludge process Biodegradable organic peroxides can be decomposed by the activated-sludge process.
(4) Use of rubber gloves and goggles is advised to avoid contact with organic peroxides which may cause damage the human skin and eyes.
Absolutely. If you need a very specific set of signage for your workplace and can’t find it in our collection, please contact us on 1300 220 536 or email us at info@safetysignsaus.com.au and tell us more about your design requirements, how many signs you need and what material you would prefer for your danger signs. We might ask you to email through images of the signs you need so that we can print them as accurately and clearly as possible. From there, we will print custom signage in our workshop in the Hunter Valley and ship them to you ASAP.
Organic peroxideproducts
(8) When a storage facility for organic peroxides is installed, legal regulations are provided for facilities, i.e., the structure and materials used for facilities as well as siting of a facility are regulated. Equipment such as an automatic charging device are required to be safe in use.
(4) Loading and unloading work for organic peroxides must be carried out at a place without fire. Smoking is prohibited during handling work.
Half-life is a convenient index which represents the decomposition rate of organic peroxides from the initial active oxygen content of the peroxide to half of that value by decomposition at a specific temperature. Half-life is measured using a solution of 0.1mol/l (occasionally 0.05mol/l) of peroxide with a solvent relatively inert to radicals, e.g. benzene, under nitrogen sealed in a glass ampoule, and immersed in a constant temperature bath set to the temperature required. Generally, decomposition of organic peroxide can be treated approximately as a first order reaction as follows:
During the polymerization of monomer and crosslinking of polymers, decomposition products of organic peroxides sometimes cause contamination, and have a bad influence on the physical properties of the polymer. Therefore, when selecting the organic peroxide, it is necessary to consider what decomposition products are generated.
(10) When an organic peroxide spills over the floor, etc., a small amount of spillage may be wiped off with a rag for burning disposal at a safe place. A large spillage requires sawdust, diatomite, vermiculite, or dry sand to absorb organic peroxide for appropriate disposal. When temporarily storing a flammable material absorbing organic peroxide, the material must be saturated with water.
Organic peroxides have various structures and decompose at different temperatures to generate free radicals. Accordingly, to choose the most suitable organic peroxides for a purpose, it is necessary to know the properties of a peroxide. Data of characteristic values such as active oxygen content, half-life, and activation energy of peroxides are important information. Moreover, organic peroxides are supplied as both the pure form and as mixtures with a diluent to increase stability. In the latter case, the effect of the diluent on conditions of polymerization must be considered. The diluent can be specified by the customer, unless it influences the stability of the organic peroxide.
Organic peroxideflammable
Organic peroxideexamples
(1) The storehouse must be cool and dark inside, and organic peroxide must be sheltered from sunlight and separated from radiators, steam pipes, and other heat sources. Organic peroxide inside the storehouse must be kept away from fire.
A stainless steel, closed-type vessel is used for tests. About 1mg of the test material is put inside the testing vessel, and is heated up at 10°C/min. The temperature at which decomposition starts (Tb: Temperature at intersectional point) and the heat of decomposition are measured.
We’re committed to making Australian workplaces safer, so we’ll start manufacturing your signs from the moment your order comes through. You will receive your danger signs within five to seven days from the order date.
Testing is carried out using a falling weight sensitivity tester which is used for explosives. The test material (0.1g) is placed on an anvil, and a roller is placed on the test material. A 5kg iron hammer falls on the top of the steel rod, and ignition or explosion of the test material is tested to observe the highest position of the iron hammer not causing ignition or explosion in ten drop tests. This method shows the sensitivity of organic peroxide against impact by a weight as well as the sensitivity to friction.
Found the appropriate signs for your workplace? Check out online with Safety Signs Australia today! We offer one of the largest collections of danger signs Australia has to offer. Get fast and free shipping on orders over $100 — your signs will be delivered within seven days of the initial order date. Choose from Colorbond Steel, hi-impact poly, aluminium or decals — we’ll print your signs right here in the Hunter Valley and provide a free laminate coating to ensure the longevity of your signs.
(2) Organic peroxide must not be stored together with other chemicals. In particular, acids such as sulfuric acid and nitric acid, amines, and metals promote extremely violent decomposition of organic peroxides. Therefore, organic peroxides must be stored separately from these materials.
(1) Burning in incinerator< A boiler as an incinerator can be used for burning disposal of organic peroxide diluted below 10% concentration (or active oxygen 1%) with inert organic solvent. However, this method is not suitable for solid type organic peroxides.
(3) When organic peroxide is swallowed The oral toxicity of organic peroxides is not exactly known. When someone has swallowed organic peroxide, it is advisable to induce vomiting after giving a large amount of milk (in the case of hydroperoxides and methylethylketone peroxides, 5% ascorbic acid sodium solution is useful) and immediately seek treatment from a physician. If the patient is unconscious, convulsed, or has a convulsive fit, vomiting should not be induced. Lay the patient on the side, maintain the mouth open for breathing and the head lower than the body, and seek immediate admission to a medical facility.
Danger/warning signs indicate that an immediate hazard or hazardous condition is likely to be life-threatening if the sign is ignored.
Active oxygen content represents not only the amount of free radicals produced from the peroxide but also the concentration or the purity of the products, i.e., the theoretical active oxygen content of 100% pure peroxide is given (Table 1) by the percentage of atomic weight of active oxygen to the molecular weight of the peroxides.
Don’t see the specific sign you need for your workplace? Get in touch with our team to organise custom signage. We can print signage based on your design and safety requirements in our Hunter Valley workshop — simply send through some images and a description of what you need, how many signs you need and what material you would like us to use to info@safetysignsaus.com.au.

(2) When organic peroxide enters the eyes Eyes must be washed with flowing water for 20 to 30 minutes without any delay, and medical treatment from a physician is necessary. (For reference, when hydroperoxides and methylethylketone peroxides enter the eyes, 5% ascorbic acid sodium solution and 3% sodium bicarbonate solution are useful for cleansing)
[Self-Accelerating Decomposition Temperature] The test material (400ml) is put in a dewar vessel, which is contained in a chamber in which air at a certain temperature is circulating. This process is carried out to investigate whether the test material decomposes, generates heat (to a temperature increase of over 6°C) under certain temperature conditions, and the rate of decomposition. A United States SADT test is conducted for the same purpose. This test uses a packaged organic peroxide product available in the market instead of tests using a small amount of organic peroxide in the dewar vessel. However, this method has the disadvantage of difficulty in use from safety and environmental pollution respects.
(5) Empty organic peroxide containers must immediately be washed with water, and the cleansed container must be safely kept uncapped in a storehouse to shield the container from sunlight until disposal.
(4) Some liquid organic peroxides gradually decompose into gas at room temperature, so containers for such peroxides are provided with a gas ejector on the lid of the container to release gas pressure inside the container. Therefore, the container must be placed with the lid side up for preventing liquid leakage. When organic peroxide is separated into another container, a gas discharge opening must be provided for the container lid.
(6) Organic peroxides are very sensitive to fire and heat, and undergo ignition and decomposition, sometimes explosion, so must be used or handled in a room away from ignition source such as electric sparking and high temperatures and heat from radiators, boilers, and other heat sources.
We have one of the largest collections of safety and danger signs Australia has to offer — from danger signs to emergency signs, workplace safety signs, fire safety signs and security signs, we have certified safety signs in stock to make your site a safer place. Browse through our collection online!
Organic peroxideformula
No, you don’t have to make an account with us to order signs. However, we recommend creating an account if you’re planning on ordering more signs/replacement signs in the future, or if you want to keep a closer eye on the progress of your order.
Danger signs signal the most serious and immediate hazards on a worksite — in fact, they are highly likely to be life-threatening. The word “DANGER” is printed in white letters inside a red oval on a black background and indicates that death or serious injury is almost certain to occur if the danger is not avoided.At Safety Signs Australia, we print high quality, highly durable danger signs right here in our workshop in the Hunter Valley of New South Wales. All of our danger/warning signs are carefully printed and designed to be AS 1319-1994 compliant, ensuring your business follows Australian Safety Standards to a tee.Based on the needs and requirements of your particular workplace, you can choose from durable materials like Colorbond Steel, aluminium, decal or hi-impact poly, and if you’re unsure which material is best suited for your workplace environment, we’ll provide expert recommendations and advice — just give us a call on 1300 220 536.We’re committed to securing Australian workplaces and keeping workers, visitors and pedestrians out of harm’s way. Explore our collection of danger signs and shop online for free shipping on orders over $100.
(1) When organic peroxide contacts the skin Repeated cleansing with soap will be sufficient. However, if itching and pain are felt after cleansing, apply an adrenocortical hormone ointment to the skin. For detailed treatment, consult a doctor.
(3) When organic peroxides are mixed with chemicals except for those described in (2), testing is required using a small volume to confirm safety. NOF is ready to give advice to users in advance.
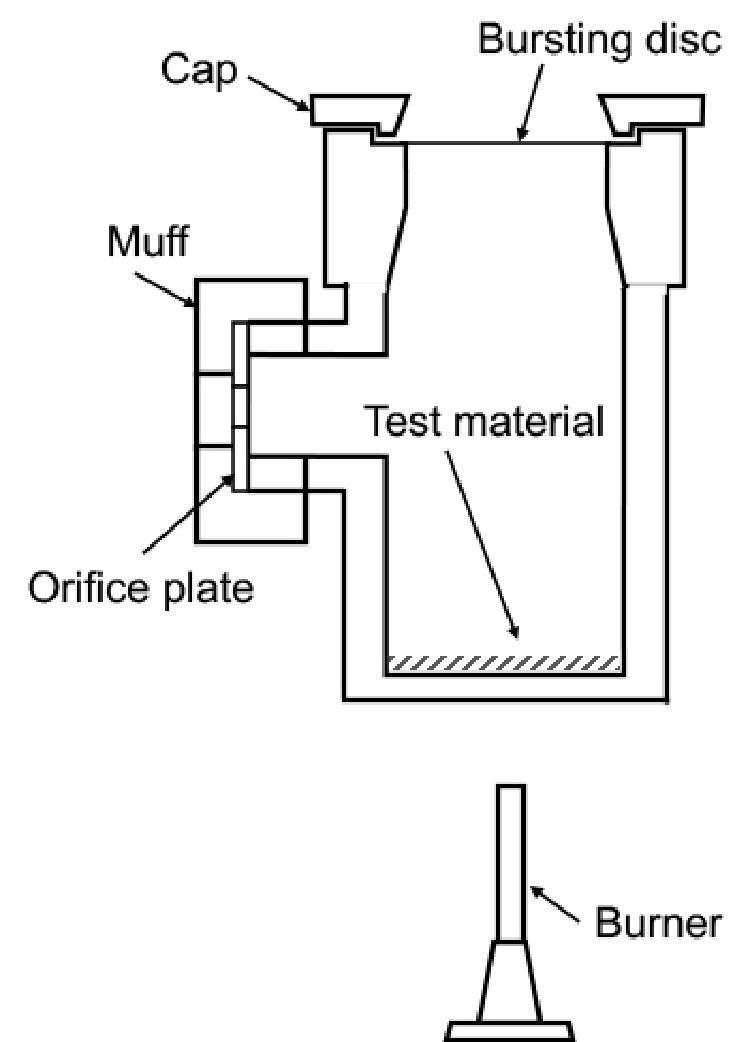
(9) Some types of organic peroxides are very sensitive to friction and shock, so friction or impact to the organic peroxide during handling must be avoided. Accordingly, ground glass is not suitable for use in containers.
The pressure vessel made of stainless steel is equipped with a bursting aluminum disc with a pressure resistance of 10kg/cm2 and a port for installing a round plate with an orifice of the selected diameter at the side of the vessel. The vessel has an inner capacity of about 235cm3, in which 5g of the test material is placed for thermal decomposition under certain conditions. The violence of decomposition power is measured from the orifice with the minimum diameter required to maintain the inner pressure at 10kg/cm2
Organic peroxides can be decomposed thermally at relatively low temperatures. They also easily produce free radicals by reaction with reducing substances. The common properties of the free radicals are the addition reaction to an unsaturated double bond and the hydrogen abstraction reaction. Based on the addition reaction, peroxides are widely used as polymerization initiators for various synthetic resins (LDPE, PVC, PS, ABS, PMMA, etc.) or as curing agents for unsaturated polyester resins, vinyl ester resins, etc. Based on the abstraction reaction, they are used as crosslinking agents for a variety of synthetic rubbers and synthetic resins, fluidity modifiers for polypropylene, and agents for grafting maleic anhydride to polyolefins.
(2) Hydrolysis Hydrolytic disposal uses a solution consisting of 80 parts water, 20 parts sodium hydroxide, and 0.3 parts surfactant, and the required solution is 10 times the weight of organic peroxide to be treated. Organic peroxide must be poured slowly into the solution. During this process, the solution should be stirred to prevent local heat increase. The solution generates heat upon decomposition of organic peroxide but there is no need to cool down the solution. Hydrolytic decomposition proceeds very slowly so stirring for 12 to 24 hours is required, and then organic peroxide is neutralized. After neutralization, separated organic substances can be recovered for burning, and/or biodegradable substances can be decomposed by the activated-sludge process.
It depends on the kind of industry you’re in and what kind of hazards/dangers there are on-site. To ensure you comply with all the latest acts, standards and regulations, we recommend contacting the following organisations in your state or territory:
The table below shows the classification by hazard of organic peroxides. Various adequate testing methods are used to assess organic peroxides for this classification at NOF. The main testing methods are briefly described below.
Organic peroxideuses
Organic peroxides are marketed under conditions with adequate care to avoid danger as described earlier but users must treat the products carefully. The handling instructions described below are principles to handle organic peroxides for safe use of the product. However, observance of the laws concerning organic peroxides is required, and the directions of the competent authorities must be followed. Please contact the NOF group about any question arising from the contents of this book.
Need custom signs? No worries. Get in touch and let us know what you need. We can print custom signs on the spot to secure the safety of your workers, visitors and passing pedestrians.
(8) When organic peroxide is treated in a closed container, a temperature monitoring device, safety valve, and a rupture disc must be provided. Residual organic peroxide in equipment and pipes must be thoroughly removed after ceasing machinery operation.
Organic peroxides may generally be considered as derivatives of hydrogen peroxides(H2O2)in which one or both of the hydrogen atoms are replaced by an organic group. Organic peroxides contain the -O-O- bond within the molecular structure, and the chemical properties of the peroxides originate from this bond.
Organic peroxidedangers
Organic peroxides that can not be used due to contamination, the guarantee period has expired, or are no longer required, must be treated with utmost care. Disposal of organic peroxides requires extensive knowledge about the properties of the specific organic peroxide to avoid occurrence of an unexpected accident. Refer to Table 3 for disposal of each organic peroxide.
At Safety Signs Australia, we design and print a wide range of danger/warning signs to keep everyone on your work site safe from harm — or even death. We offer:
(2) Organic peroxides must not be directly mixed with compounds containing iron, cobalt, manganese, etc., because these compounds cause redox reaction with organic peroxides, and promote the decomposition of organic peroxides. Direct mixing of organic peroxides with amine compounds must also be avoided. When these compounds are used, organic peroxides and these compounds must be separately diluted with resin monomer. After dilution, either may be mixed with the other.
Therefore, organic peroxide decomposition at a specific temperature has a linear relationship between time (t) and [ln a/(a-x)], so (k) can be obtained from the slope of the straight line. Then, half-life (t1/2) can be obtained at the specific temperature from equation (3). The rate constant (k) is given as follows:
Yes. “Danger” is used for more severe, life-threatening hazards whereas “warning” is used for less severe dangers and hazards. “Warning” signs are for hazards that are potentially dangerous.
A 500ml flask is placed in a electric furnace with the temperature controlled adequately, and the test material (0.15ml) is put in the flask. In this method, the lowest temperature that induces ignition is measured to decide the ignition temperature (auto-ignition temperature). Organic peroxides undergo thermal decomposition, and almost all organic peroxides show rapid decomposition accompanied by white smoke before ignition.
If a fire involving organic peroxide starts, or there is a fear of igniting organic peroxide, the fire extinguishing method varies according to the surrounding environment at the fire place.
Measurement of (k) at several temperatures provides the relationship between (lnk) and (1/T), so activation energy can be obtained from the slope of the straight line. The straight line relationship of (lnt1/2) to (1/T) can be used to find the half-life of organic peroxide at any temperature, or the decomposition temperature required for a particular half-life.
(Tag type, Setaflash type, and Cleveland type) The flash point is defined as the temperature at which ignition of the test material is observed by an open flame over the test material heated to a certain temperature.
The tester is equipped with a friction rod and plate made of porcelain. A small amount of the test material is placed between the rod and plate. Friction motion is caused under a load, and the sensitivity of the organic peroxide is studied from the relationship between load and explosion.
In general, organic peroxides are unstable to heat, and some types of organic peroxides are very sensitive to friction and shock, and decompose explosively. On the other hand, other types can be handled in a similar way to solvents. Accordingly, handling of organic peroxides requires adequate knowledge of the characteristics of each organic peroxide. Therefore, it is important to handle organic peroxides by appropriate procedures for safe and efficient use. Adequate assessment techniques for the characteristics of organic peroxides are required, and various testing methods have been developed for this purpose. Based on assessment tests, organic peroxides can be classified into various grades of hazard. The NOF group also has basic data on handling organic peroxides, obtained from various tests conducted at NOF.
(11) To handle organic peroxides requiring temperature control, the necessary amount of organic peroxide must be subdivided, and used immediately. The remaining organic peroxide must be returned to a temperature-controlled storehouse. Organic peroxide reaching room temperature during the period outside the storehouse must not be returned to the storehouse as it is. Organic peroxide reaching a temperature higher than the required storage temperature must be abandoned, or cooled in a water bath below 5°C for return to the storehouse.
A number of environmental factors on-site will determine what kind of sign materials you need. We offer Colorbond Steel, hi-impact poly, aluminium and decal — here’s a quick rundown of the different benefits and ideal worksites.
(3) Careful handling and transportation are required for organic peroxides, to avoid friction and vibration, or dropping and falling.
(7) Equipment materials in contact with organic peroxides must be selected from stainless steel, glass lining, glass, and polyethylene. Gaskets made of chemical-resistant materials such as Teflon (polytetrafluoroethylene) are also allowed.
(1) Thorough care is required to prevent contamination of organic peroxides during handling because contamination may promote decomposition of organic peroxides. When organic peroxides are subdivided to small volumes for use, glass, stainless steel (SUS304 or SUS316), or polyethylene containers must be used. Containers made of steel, copper alloys, lead, rubber, etc. are not useful. Moreover, the subdivided organic peroxide must not be returned to the original container. Containers and vessels containing subdivided organic peroxide must be clearly labeled.
The test material (1g) is put in a test tube, and heated using an electric heating plate at an increasing rate of 4°C/min. to observe the temperature at which the test material starts decomposing and decomposition status.
There are various types of organic peroxides, e.g. refrigerated organic peroxides undergoing self-accelerating thermal decomposition below room temperature, stable organic peroxides which do not generate appreciable amounts of free radicals without being heated to high temperatures, and/or some peroxides which generate radicals reacting with an accelerator.
Organic peroxides may generally be considered as derivatives of hydrogen peroxides (H2O2) in which one or both of the hydrogen atoms are replaced by an organic group. Organic peroxides contain the -O-O- bond within the molecular structure, and the chemical properties of the peroxides originate from this bond.
Testing is carried out using a steel block and the No.6 blasting cap in a rigid enclosure. The blasting cap explodes (or decomposes) 10g of the sample. The deflection angle of the weight is compared with a reference of TNT as 100%. This indicates the explosive power of the sample.
Half-life (t1/2) is the time required to reduce (a) to (a/2) by decomposition, so equation (3) can be obtained by substituting (a/2) for (x) in equation (2).
If you’re unsure which material is right for your workplace, please give us a call on 1300 220 536 or email us at info@safetysignsaus.com.au. We can provide recommendations and advice based on years of experience in the signage industry and our thorough understanding of Australian Safety Standards. We will ensure you have the best possible, longest lasting signage for your workplace.
Danger/warning signs are required in chemical storage areas, restricted areas, radiation risk areas, workplaces with falling hazards, high voltage areas, workplaces with flammable gasses and explosion risks, confined spaces, construction sites, workplaces with buried power cables, workplaces with heavy machinery and more.









 13322766566
13322766566